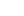
2-(4-(Aminomethyl)phenoxy)-N,N-Dimethylethanamine
Chenglian Pharmaceutical is an innovative high-tech pharmaceutical enterprise that is market-oriented and realizes industrial development through R&a¶mp;D innovation and technology transformation.
key word:
Category:
Product Description
Integrity, innovation, cooperation, sharing, win-win
The company integrates multiple resources and strives to build a large health industry∞ finance platform company integrating pharmaceutical, intelligent medical, b∑io pharmaceutical R&D, industrial production and sales.
The chemical reactions involved in the existing products include Foucault reaction, nitra÷tion reaction, sulfonation reaction, hydrogenation reaction, fluorination reaction, chlorination☆ reaction, bromination reaction, diazotization reaction, format reaction, etc.
Related products
Sitagliptin Phosphate Monohydrate
Upadacitinib(Scientific research)
Product Consulting

Follow WeChat
Anhui Union Pharmaceuticals Technology Co., Ltd.
Service line:
+86-21-54285906
E-mail:2880705932@qq.com
Add:4th Floor, Building B3, Creative Industry Park, Hefei, Anhui P. R. China.
Copyright © 2022 Anhui Union Pharmaceuticals Technology Co., Ltd.