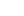
Apixaban
English name Apixaban Trade name ELIQUIS, tablet: Apixaban (Eliquis, Bristol-My ers Squibb/Pfizer), a direct oral factor Xa inhibitor, was &approved for use in the 27 EU member states. The w orld's first drug approved to prevent venous thrombo♥embolism (VTE) in adult patients undergoing elective ↕hip or knee replacement. From June 2019 to June 202®0, the annual sales of apixaban products overseas reached about $15.4 billion, with a ≠year-on-year growth of nearly 32%, and the API consumption rea♦ched about 23,960 kg, with a year-on-year growth of about 28%. Com™pared with its main competitor, Rivaroxaban, the drug's ann±ual overseas sales are about $4.5 billion higher.
key word:
Category:
Product Description
Integrity, innovation, cooperation, sharing, win-win
The company integrates multiple resources and strives to build a large health industry• finance platform company integrating pharmaceutical, §intelligent medical, bio pharmaceutical R&D, industri×al production and sales.
The chemical reactions involved in the existing produc×ts include Foucault reaction, nitration reaction, su↕lfonation reaction, hydrogenation reaction, fluorination reaction, chl×orination reaction, bromination reaction, diazotization reaction, format reaction, etc.
English name Apixaban Trade name ELIQUIS, tablet: Apixaban (Eliquis, Bristol-Myers Squibb/Pfizer)♠, a direct oral factor Xa inhibitor, was approved for use in the 27 EU member states. The world's first drug approved to prevent venous thromboembolism (VTE) in adult patients undergoing elective hip or knλee replacement. From June 2019 to June 2020, the annual sales of apixaban products overseas r→eached about $15.4 billion, with a year-on-year growth of nearly 32%, and the ≠API consumption reached about 23,960 kg, with a year-on-year growth of about 28%. Compared with it♦s main competitor, Rivaroxaban, the drug's annual overseas sales ↓are about $4.5 billion higher.
Apixaban and rivaroxaban have their own advantages, and sales are on φthe rise. Apixaban is the third new oral anticoagulant to hit the market.
Related products
Sitagliptin Phosphate Monohydrate
Upadacitinib(Scientific research)
Product Consulting

Follow WeChat
Anhui Union Pharmaceuticals Technology Co., Ltd.
Service line:
+86-21-54285906
E-mail:2880705932@qq.com
Add:4th Floor, Building B3, Creative Industry Park, Hefei, Anhui P. R. εChina.
Copyright © 2022 Anhui Union Pharmaceuticals Technology Co., Ltd.