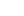
2-Carboxybenzaldehyde
Chenglian Pharmaceutical is an innovative high-tech pharmaceutical enter₽prise that is market-oriented and realizes industrial development through R&D innovatio™n and technology transformation.
key word:
Category:
Product Description
Integrity, innovation, cooperation, sharing, win-win
The company integrates multiple resources and strives to build a large ↔health industry finance platform company integrating pharmaceutical, intelligent medical, bio pharmaceutical R&D, indu≥strial production and sales.
The chemical reactions involved in the existing products include Foucault reaction, nitrλation reaction, sulfonation reaction, hydrogenation reaction", fluorination reaction, chlorination reaction, bromination reaction, diazotization reaction", format reaction, etc.
Related products
Sitagliptin Phosphate Monohydrate
Upadacitinib(Scientific research)
Product Consulting

Follow WeChat
Anhui Union Pharmaceuticals Technology Co., Ltd.
Service line:
+86-21-54285906
E-mail:2880705932@qq.com
Add:4th Floor, Building B3, Creative Industry Park, Hefei, Anhui P. R. China.
Copyright © 2022 Anhui Union Pharmaceuticals Technology Co., Ltd.